- Habit 1:
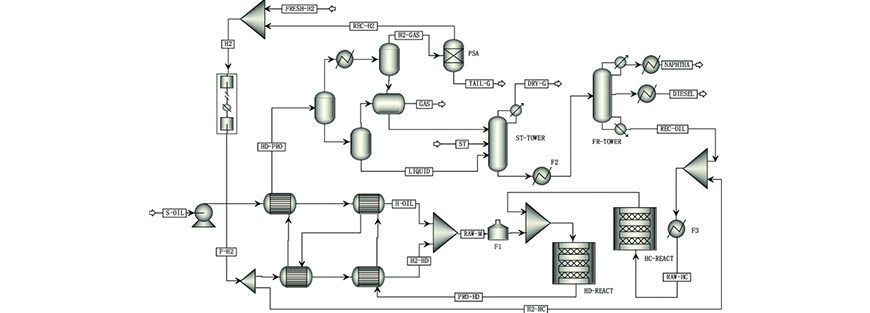
You build a simulation model to meet an objective: The purpose of running a simulation should be clearly defined upfront. For instance, if one were to perform mass and energy balances for a preliminary flowsheet, it may be acceptable to use simplified unit operation models available in the simulation software. A good example is the use of a shortcut distillation model that uses the Fenskee-Underwoode-Gilliland method to provide a first-pass distillation model before constructing a rigorous tray-by-tray distillation model. The shortcut model would normally require the definition of light and heavy keys, top and bottom column pressures, along with reflux ratio to converge the column model. The Rigorous model requires some detailed information, such as the number of trays, top and bottom temperatures.
- Habit 2:
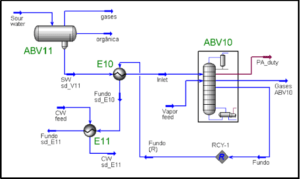
Identify the system or process and draw an envelope around it: It is important to identify the system that we wish to simulate. In some cases, not the entire flowsheet will be simulated. This could be due to several reasons. One typical example is the limitation of the simulation software.
- Habit 3:
Imagine what is going on physically: The engineers who perform simulation tasks should have a good imagination. For instance, they should imagine the state and flow pattern/regime for an inlet heading to a reactor/flash unit or an effluent emitted from a reactor.
- Habit 4:
Translate the physical model to a mathematical model: Engineers should use their basic knowledge in reaction engineering, thermodynamics, separation processes, etc., to translate a physical process in the plant into an equivalent mathematical model. The advice is to use the right software for the right applications.
- Habit 5:
Know your components: It is important to know the chemicals that are present in the system you are working on. Furthermore, it is important to understand the type of intermolecular interactions that may exist in that system. Understanding the components and their interactions will enable them to choose the right property estimation methods. In some cases, there may be some components that are not represented in the simulator’s component database. In that case, it may be necessary to either find an equivalent component or create a user-defined or “hypothetical” component. One will also have to be aware of the presence of in a predominantly hydrocarbon process. Some thermodynamic models may have to lump the organic and aqueous phases into a single liquid phase, even though is immiscible with the hydrocarbon. Hence, the selection of the thermodynamic model is crucial for this kind of system.
- Habit 6:
Know the context of your feed streams It is important to know the characteristics of the feed, e.g., its origin, composition, sampling conditions, and the presence of impurities. In some cases, a composition for a gas that comes from a three-phase (gas, oil, and) separator may not contain any. This is because the composition has been determined using gas chromatography (GC). In this case, it is necessary to saturate the gas before using it for any computations; otherwise, the contribution of in the gas, particularly the heat of vaporization, will not be accounted for and will result in an inaccurate heat and material balance.
- Habit 7:
Know your components boiling points In performing simulation for a separation system (e.g., flash and distillation), it is important to know the boiling points, and hence the relative volatility of the chemicals involved. It is good practice to have the chemicals arranged in the ascending of their boiling points (i.e., volatilities). Besides, we should also watch out for polar molecules and those chemicals that have hydrogen bonds. This may give rise to azeotropic systems where high boiling point components boil and vaporize before low boiling point components.
- Habit 8:
Keep track of the units of measure in all calculations Errors on units of measurement are very serious and the easiest errors to avoid with some simple discipline. The best practice is always to keep track of the units of measurement in all computation or simulation exercises.
- Habit 9:
Always do a simple material and energy balance first An important good habit is to perform some manual calculations (or “hand calculations”). For example, performing quick material and energy balances before executing a process simulation exercise enables them to have a better “feel” for the orders of magnitude in the numbers that may be encountered. In addition to that, these hand calculations can also be used as initial guesses for more complex types of computations.
- Habit 10:
Plot the phase envelope for important streams It is extremely important to know in which state the fluid is in, e.g., is it a subcooled or saturated liquid? For a process with a subcooled liquid, the temperature rise is expected when sensible heat is added; the latter is the product of mass flow rate, heat capacity, and temperature rise. On the other hand, no temperature rise will be reported when a saturated liquid is heated, as latent heat is involved. Also, it is necessary to if the fluid is near the critical point, or is it at the supercritical stage? It is also important to know how close the process is to the dew point (either hydrocarbon or). When adsorption beds or fuel gas systems were designed, liquids formed at the dew point may damage the adsorption bed or combustion chamber. Another important practice is to identify the retrograde region of the fluid. In the retrograde region, compressing a fluid may result in its vaporization instead of liquefaction.